by Gary Ciment, Ph.D., Scientific Director, Aves Labs, Inc.
Zika Virus (ZIKV) is a mosquito-transmitted member of the flavivirus family, and has been implicated in recent human epidemics in Africa, Southeast Asia, and South and Central America. ZIKV infection of pregnant women produces severe fetal abnormalities, including microcephaly and death. In non-pregnant woman and in men, ZIKV infection has been shown to produce fever, muscle and joint pain, headache, and in rare instances, Guillain-Barré syndrome, a potentially-deadly neuromuscular condition. In this regard, the ZIKV seems to be tropic towards the central nervous system (CNS), as well as other organs.
Although the mechanism(s) by which flavivirus infection results in these symptoms is incompletely understood, recent evidence suggests that one of the gene products encoded by the ZIKV interferes with type I interferon (IFN-I) signaling. Viral antagonism of this signaling pathway is important for Zika virus to disseminate from the initial site of infection to reach the placenta and developing fetus. In particular, the non-structural viral protein NS5, which encodes an RNA-dependent RNA polymerase, has been shown to bind to and antagonize STAT2, a latent transcription factor that becomes activated after cells are stimulation with type I interferon and functions to drive gene activation (Grant et al., 2016; Kumar et al., 2016).
To examine the newly emerging Zika virus and its impact on the IFN-I response, Dr. Sonja Best (Laboratory of Virology, Rocky Mountain Laboratories, National Institute of Allergy and Infectious Diseases, Hamilton, MT) contacted us several years ago to make ZIKV NS5-specific antibody. One of the goals of this antibody was to investigate the neurotropic properties of ZIKV and the cell types that ZIKV infects in the brain. In particular, she wanted an antibody that could easily be used in immunohistochemical studies with other commercial antibody markers of neural cells, mostly made using rabbit and mouse hosts. For this reason, chicken antibodies would be an ideal reagent for these studies, since secondary antibodies against the chicken IgY do not crossreact with mammalian IgG marker antibodies, and vice versa. One could easily use chicken antibodies, therefore, together with mammalian IgG's, in various multiplex assays.
Using our proprietary Immunogenicity Algorithm®, we identified a number of peptide sequences within the ZIKV NS5 gene product (NCBI, YA_009227205.1), from which Dr. Best chose one sequence near the C-terminus -- DEE KYM DYL STQ VRY LGE E (residues 878-896). Following our standard injection protocol, a KLH-conjugate of this peptide was injected over a 7-week period into the breast muscles of two laying hens, and then eggs were collected over a 3-week period after the 4th injection. From 12 of these eggs, we purified about 1500 mg of IgY using our proprietary methods; from this IgY preparation, we further purified 5.5 mg of affinity-purified antibody. Since the hens were unharmed during the egg collection phase of the project, we performed additional injections and additional purifications of antibody. In the end, we were able to purify almost 40 mg of affinity-purified antibody against NS5 from about 72 eggs.
Using Rag1-/- adult mice (which lack a critical factor in B-cell maturation, and therefore, allow ZIKV to infect normally virus-resistant wild-type mice), Dr. Best and her collaborators found ZIKV NS5 immunoreactivity to be wide-spread within the brain. In particular, NS5 immunostaining seemed to be specific to neurons, and not other cells of the CNS. Figure 1 below (from Figure 7 of the Winkler et al, 2017 paper) shows NS5-immunoreactivity (green fluorescence) in neurons of the CA1 region of the hippocampus, as identified by the neuron-specific nuclear antigen NeuN (i.e., a.k.a. "Fox3," pseudo-magenta fluorescence in panel A). In panel A of this photomicrograph, you can see NeuN immunostaining of nuclei (green) surrounded by NS5 immunostaining of the cytoplasm of these hippocampal neurons (i.e., false magenta staining). In contrast, NS5 immunoreactivity was not seen in astrocytes (identified by GFAP-immunoreactivity, pseudo-magenta fluorescence, panel E) or in microglial cells (identified by Iba1-immunoreactivity, pseudo-magenta fluorescence, panel I).
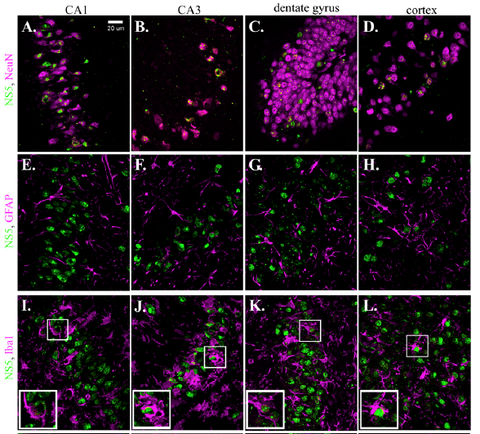
This pattern of neuron-specific staining pattern can also be seen in other parts of the brain. This same figure shows neuron-specific NS5 immunoreactivity in neurons of the CA3 region of the hippocampus (panels B, F, J), dentate gyrus (panels C, G, K) and cerebral cortex (panels D, H, L). These result show that, at least within the CNS, ZIKV-tropism for neurons is wide spread.
It should be noted that treatment of wildtype C57/B6 mice show similar patterns of NS5 immunostaining when an antibody neutralizing the type I Interferon signaling pathway is used instead of the Rag1-/- mutant mice. This indicates that this ZIKV tropism for neurons within the CNS is not unique to this mutant mouse line, but is instead a general feature of ZIKV infectivity (Smith et al., 2017).
These and other data suggest that ZIKV selectively infects neurons of the CNS. Virus replication in neurons eventually kills them, and it is presumably aided by interfering with an intracellular signaling pathway that is critical for the antiviral response stimulated by type I interferon. Antiviral drugs that disrupt the interaction between STAT2 and NS5 may be useful in treatment of ZIKV infection by enabling the antiviral functions of type I interferon as part of the normal immune response.
References
- Darci R, Hollidge B, Daye S, Zeng X, Blancett C, Kuszpit K, Bocan T, Koehler JW, Coyne S, Minogue T, Kenny T, Chi X, Yim S, Miller L, Schmaljohn C, Bavari S, Golden JW. (2017). Neuropathogenesis of Zika Virus in a Highly Susceptible Immunocompetent Mouse Model after Antibody Blockade of Type I Interferon. PLOS Negl Trop Dis 11(1): e0005296. doi:10.1371.
- Grant A, Ponia SS, Tripathi S, Balasubramaniam V, Miorin L, Sourisseau M, Schwarz MC, Sanchez-Seco MP, Evans MJ, Best SM, Garcia-Sastre A. (2016). Zika Virus Targets Human STAT2 to Inhibit Type I Interferon Signaling. Cell Host & Microbe 19: 882-890.
- Kumar A, Hou S, Airo AM, Limonta D, Mancinelli V, Branton W, Power C, Hobman TC. (2016). Zika Virus Inhibits Type-I Interferon Production and Downstream Signaling. EMBO Rep 17: 1766-1775.
- Smith DR, Hollidge B, Daye S, Zeng X, Blancett C, Kuszpit K, Bocan T, Koehler JW, Coyne S, Minogue T, Kenny T, Chi X, Yim S, Miller L, Schmaljohn C, Bavari S, Golden JW. (2017). Neuropathogenesis of Zika Virus in a Highly Susceptible Immunocompetent Mouse Model after Antibody Blockage of Type I Interferon. PLOS Negl Trop Dis 11(1): e0005296.
- Winkler CW, Myers LM, Woods TA, Messer RJ, Carmody AB, McNally KL, Scott DP, Hasenkrug KJ, Best SM, Peterson KE. (2017). Adaptive Immune Responses to Zika Virus Are Important for Controlling Virus Infection and Preventing Infection in Brain and Testes. J. Immunol. 198: 3526-3535.


